-
PMC5036995

Published online 2016 Mar 30. doi: 10.1016/j.cardfail.2016.03.013
Prognosis of Low Normal Left Ventricular Ejection Fraction in an Asymptomatic Population-based Adult Cohort. MESA
Abstract
Background
Reduced left ventricular systolic function predicts worse outcomes. However, the optimal threshold for “normal” left ventricular ejection fraction (LVEF) is uncertain. In general LVEF ≥ 55% is considered “normal” by guidelines, with a low normal designation for LVEF 50-55%. We assess the prognosis of participants with low normal LVEF in the Multi Ethnic Study of Atherosclerosis. All participants were asymptomatic and had no known clinical CVD at baseline.
Methods and Results
4926 out of 6814 had LVEF assessed using cardiac MRI, had no significant valvular disease, did not have MI during follow up, had complete data and included in this analysis. 83/4926(1.7%) had LVEF <50% (low LVEF) and 101/4926(2.1%) had low normal LVEF. Cox proportional hazard and cubic spline analyses were used to evaluate the association between LVEF category and 10 years of adjudicated incident congestive heart failure (CHF) and all- cause mortality adjusting for age gender and race (model 1) and model 2: model 1 + DM, smoking, SBP, BP meds, BMI, eGFR, LDL, family history of CHD, educational status and LV mass.
Mean age 61± 10yrs, 47% men, 35% on BP meds, 9% diabetes. After 10.2 years of follow up, 109(2.2%) had CHF and 427(8.7%) died. Compared with normal LVEF (≥55%), low normal LVEF and low LVEF were associated with an increased risk for incident CHF during follow up in our multivariable Cox models [Hazard ratio (95% CI): 3.64(1.76-7.52) and 9.52(5.63-17.52) respectively. Unlike low LVEF, Low normal LVEF was not associated with increased risk of death compared with normal LVEF in our fully-adjusted models [hazard ratio (95%CI): 3.03(1.94-4.73) and 1.32(0.72-2.41) respectively. The adjusted spline analysis HR of LVEF =55% as reference, LVEF had a U shape association of future CHF risk and LVEF
Conclusion
Low normal LVEF is as prevalent as low LVEF in asymptomatic community dwelling adults. We observed a gradient-response association between the three categories of LVEF (low, low normal and normal LVEF) and incident CHF but not for all cause death.
Introduction
Heart failure is a leading cause of morbidity and mortality in the world today despite decades of research1, 2. Current data suggests that while the prevalence of heart failure with reduced ejection fraction (HFref) seems to have plateaued or on the decline, the prevalence of heart failure with preserved ejection fraction (HFpef) is rising3, 4. The apparent decline in HFref prevalence is in part due to the improvement in the management and prevention of coronary artery disease and its sequelae3. However, the prevalence of HFref due to causes other than coronary artery disease (non-ischemic cardiomyopathy), appears to be the same or increasing especially in non-white ethnicities and prevention maybe the best approach to combat it 5–7.
The American Heart Association (AHA) introduced a new heart failure classification to help identify the primordial stage (stage A), subclinical (stage B) and the clinical (stage C&D) stages of the disease for prevention and treatment1. AHA Stage B heart failure denotes individuals with structural heart disease which includes asymptomatic low left ventricular ejection fraction. For the AHA stage B HF individuals proven targeted therapies such as angiotensin converting enzyme inhibitors(ACE-I) and beta blockers are recommended in addition to non-targeted therapies such as lifestyle changes 1, 8. In general clinical practice, left ventricular ejection fraction (LVEF) ≥ 55% is considered normal and LVEF 50-55% is designated as “low normal”. As written, the AHA stage B heart failure excludes individuals with low normal LVEF unless they have a history of myocardial infarction, left ventricular hypertrophy or significant valvular heart disease8. This may be due to the paucity of data on the prevalence, prognosis and the effects of current heart failure preventive therapy in such asymptomatic individuals in our communities.
In this report, we used baseline Cardiac Magnetic Resonance imaging data of participants in the ongoing Multi Ethnic Study of Atherosclerosis (MESA) and 10 years of adjudicated congestive heart failure and death, to assess the prevalence and prognosis of asymptomatic individuals low normal LVEF.
Methods
Study Population and Data Collection
A detail study design for MESA has been published elsewhere9. In brief, MESA is a prospective cohort study begun in July 2000 to investigate the prevalence, correlates, and progression of subclinical CVD in individuals without known CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). MESA participants were 38% white (n = 2624), 28% black (n = 1895), 22% Hispanic (n = 1492) and 12% Chinese (n = 803). Individuals with a history of physician-diagnosed myocardial infarction, angina, heart failure, stroke or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded. This study was approved by the Institutional Review Boards of each study site and written informed consent was obtained from all participants.
Demographics, medical history, anthropometric and laboratory data for this study were obtained at the first MESA examination (July 2000 to August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus was defined as fasting glucose ≥126 mg 100 ml−1 or the use of hypoglycemic medications. Use of antihypertensive and other medications was based on the review of prescribed medication containers. Resting blood pressure was measured three times in seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg)/height (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-h fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation10.
Cardiac Magnetic Resonance Imaging
Consenting participants underwent a cardiac MRI scan a median of 16 days after the baseline evaluation; 95% were completed by 11 weeks after the baseline examination. Participation in the MRI exam was voluntary. All imaging was done with a four-element phased-array surface coil positioned anteriorly and posteriorly, electrocardiographic gating, and brachial artery blood pressure monitoring11. Imaging consisted of fast gradient echo cine images of the left ventricle with time resolution < 50 ms. Functional parameters and mass were determined by volumetric imaging. Imaging data were read using MASS software (version 4.2, Medis, Leiden, the Netherlands) at a single reading center by trained readers blinded to risk factor information. Papillary muscles were included in the LV volumes and excluded from LV mass. LV end-diastolic volume and LV end-systolic volume were calculated using Simpson’s rule (the summation of areas on each separate slice multiplied by the sum of slice thickness and image gap). LV mass was determined by the sum of the myocardial area (the difference between endocardial and epicardial contour) times slice thickness plus image gap in the end-diastolic phase multiplied by the specific gravity of myocardium (1.05 g/mL). LVEF was calculated as LV stroke volume/ LV end-diastolic volume × 100. The interobserver variability in estimating LV parameters was: LVEF (5.1%, 95% CI 3.6, 6.7) and intraobserver variability in estimating LV parameters was: LVM (6.3 gm, 95% CI, 5.17, 7.38); LVEF (3.9%, 95% CI, 3.06, 4.72).
Ascertainment of Outcomes
Outcomes in MESA are adjudicated by a committee which includes a cardiologist, a cardiovascular physician-epidemiologist and a neurologist. Reviewers/ adjudicators classified incident CHF as definite, probable, or absent. Definite or probable CHF required heart failure symptoms, such as shortness of breath or edema; probable CHF required CHF diagnosed by a physician and patient receiving medical treatment for CHF. Definite CHF required one or more other criteria, such as pulmonary edema/congestion by chest X-ray; dilated ventricle or poor LV function by echocardiocardiography or ventriculography; or echocardiography evidence of left ventricular diastolic dysfunction. Individuals with adjudicated definite or probable CHF were used in our analysis. All –cause mortality (death) was also adjudicated by committee.
MESA Participants Included in this study (N=4926)
All MESA participants with significant valvular heart disease during baseline exam or had an adjudicated myocardial infarction during the 10 years of follow up were excluded from this analysis. For this analysis low LVEF and low normal LVEF (LN) were defined as participants with baseline LVEF of <50% and 50-55% respectively.
Statistical Analysis
Demographic characteristics of participants with low, low normal and normal LVEF are reported as mean ±SD for continuous variables and as frequency or percentages for categorical variables. Analysis of variance (ANOVA) and Chi square analysis were used to compare baseline variables among the three LVEF groups (Low, Low normal and normal LVEF).
Kaplan-Meier analysis was used to explore the association between low, low normal and normal LVEF with incident CHF. Cox proportional hazard analysis was also used to assess the association between low, low normal and normal LVEF and outcomes (incident CHF, all-cause mortality) in univariable and 2 multivariable models: Model 1: Adjusted for age, sex, race; Model 2: Model 1 + diabetes, cigarette smoking status, hypertension medication use (ACEI and beta-blocker use), systolic blood pressure, hyperlipidemia, family history of CHD, education status, estimated glomerular filtration rate(eGFR), body mass index and left ventricular end diastolic mass. These covariates were selected based on their association with CHF in prior studies and in the present study in univariable analysis. The above analyses were repeated with all-cause death as the outcome of interest. Adjusted cubic spline analysis with knot at LVEF of 50%, 55% and 65%, was used to assess the risk of increasing LVEF from 50% upwards for future CHF events with LVEF = 55% as the reference. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Out of the 5004 MESA participants who had CMRI during the baseline exam, 4926 met the inclusion criteria and also had complete data. The mean age (SD) of this cohort was 61± 10 yrs., 47% males, 39% whites, 26% African Americans, 13% Chinese, 22% Hispanics and 9% had diabetes. 101/4669(2.1%) had low normal LVEF. After a median of 10.2 years, 109(2.2%) had adjudicated CHF and 427(8.7%) died. Table 1 shows the demographic characteristics, clinical history and laboratory variables of participants in this study stratified by LVEF category. Participants with low LVEF were older, more likely to be males, have diabetes mellitus and current smoker compared with low normal and normal LVEF participants. Participants with low normal LVEF were also more likely to be males compared with those with normal LVEF. Participants with normal LVEF had significantly higher HDL levels compared with those with low LVEF. Participants with low LVEF had higher LV end diastolic mass compared with those with low normal and normal LVEF. Participants with low normal LVEF also had significantly higher LV end diastolic mass compared with those with normal LVEF (Table 1).
Table 1
Clinical Characteristics of the Study Cohort (N = 4,926)
|
|
|
|
|||
|---|---|---|---|---|---|
| Variables | All cohort (n = 4926) | Low EF (n = 83) | Low normal (n = 101) | Normal EF (n =4742) | P |
|
|
|
|
|||
| Demographics | |||||
| Age (years) | 61.5±10.1 | 62.7±10.6 | 61.3±9.4 | 61.5±10.3 | 0.01 |
| Men | 47.2% | 83.1% | 71.3% | 46.0% | <0.0001 |
| Race/Ethnicity | |||||
| Caucasian | 39.1% | 38.6% | 39.6% | 37.6% | <0.0001 |
| African American | 25.8% | 38.6% | 39.6% | 25.3% | |
| Asians | 13.1% | 1.2% | 2.0% | 13.5% | |
| Hispanics | 22.0% | 21.7% | 18.8% | 22.1% | |
| Education >graduate | 19.6% | 11.2% | 24.8% | 19.6% | 0.19 |
| Clinical History | |||||
| Hypertension medication use | 35.2% | 32.5% | 38.6% | 33.9% | 0.68 |
| Diabetes | 8.9% | 16.9% | 11.9% | 8.6% | 0.03 |
| Cigarette smoking | |||||
| Current | 12.6% | 27.5% | 9.9% | 12.4% | <0.0001 |
| Former | 35.8% | 45.0% | 45.5% | 35.4% | <0.0001 |
| Hyperlipidemia | 15.8% | 14.5% | 8.9% | 16.0% | 0.11 |
| Family history of CHD | 33.1% | 34.9% | 37.6% | 33.0% | 0.06 |
| Laboratory Variables | |||||
| Cholesterol (mg/dL) | 194.2±35.5 | 190.7±40.2 | 194.2±35.3 | 194.2±35.3 | 0.36 |
| HDL (mg/dL) | 51.3±15.0 | 47.0±13.0 | 49.8±13.8 | 51.6±15.0 | <0.0001 |
| Body mass index (kg/m2) | 27.7±5.0 | 28.2±4.9 | 28.3±5.3 | 27.7±5.0 | 0.72 |
| Systolic blood pressure (mmHg) | 125.4±21.3 | 129.4±21.8 | 127.3±21.5 | 125.2±21.2 | 0.17 |
| LV end diastolic Mass(gm) | 121.0± 30.0 | 162.9± 41.0 | 149.9 ±35.2 | 119.5± 28.6 | <0.001 |
N (%) and mean ± SD given in the table. LV: left ventricular
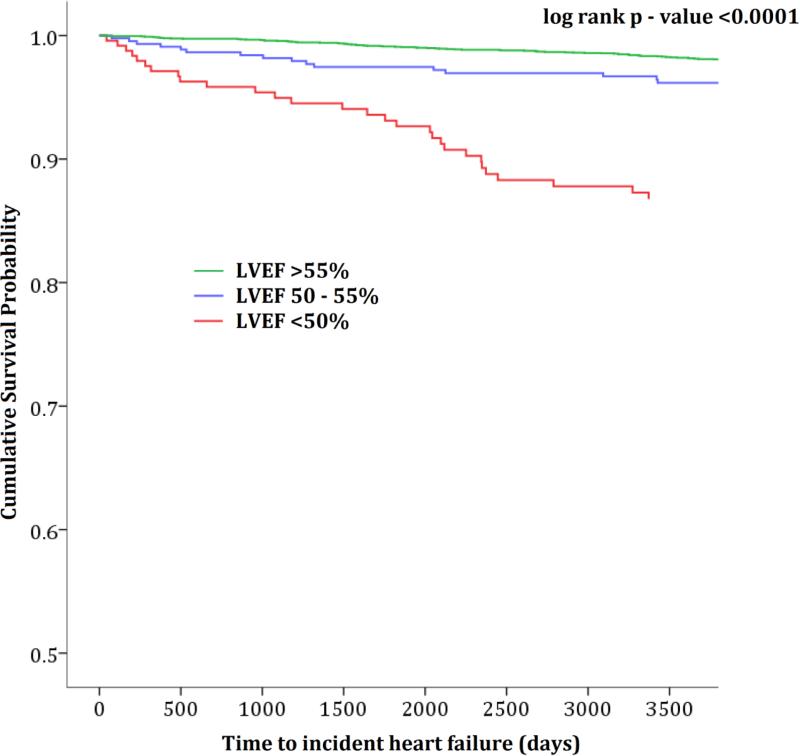
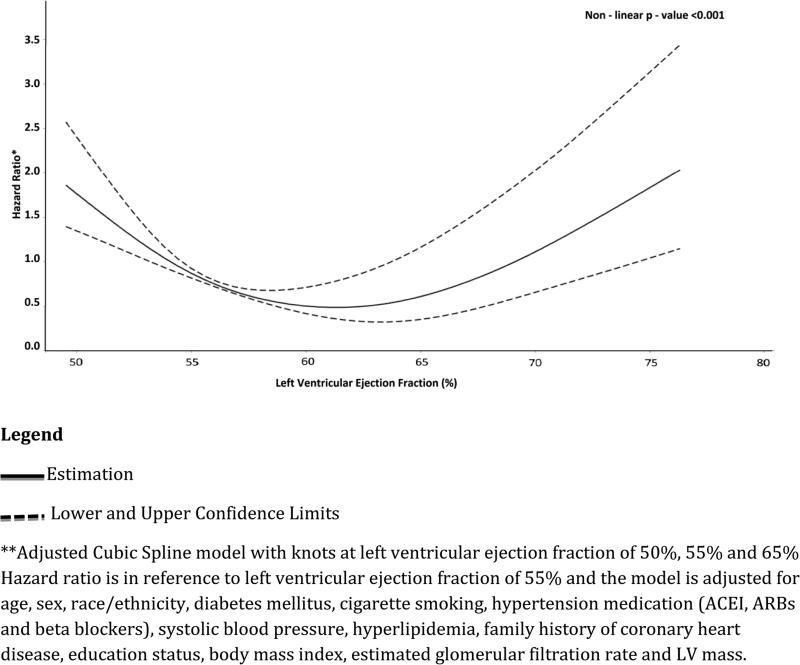
Both low and low normal LVEF were independently associated with increased risk for incident CHF in our univariable and multivariable models (Figure 1 and Table 2). Compared with normal LVEF (reference group), low normal LVEF as associated with incident CHF in unadjusted [HR (95%CI): 3.58(1.74-7.33)], after adjusting for age, sex and race/ethnicity [HR (95%CI): 3.57(1.74-7.34)] and in our full multivariable Cox model [HR (95%CI): 3.64(1.76-7.52)]. Compared with normal LVEF, low LVEF was also associated with incident CHF in unadjusted [HR (95%CI): 13.31(8.17-17.33)], after adjusting for age, sex and race/ethnicity [HR (95%CI): 11.34(6.87-18.91)] and also in our full model [HR (95%CI): 9.52(5.63-17.75)]. We observed a gradient-response association; individuals with low normal were > 3 times while those with low LVEF were > 9 times more likely to develop CHF during the follow up period compared with those with normal LVEF. Compared with participants with normal LVEF, low normal LVEF was not associated with increased risk of all-cause mortality in either the unadjusted, after adjusting for age, sex and race/ethnicity or the full multivariable model [HR(95%CI): 1.21(0.66-2.19), 1.23(0.68-2.24)) and 1.32(0.72-2.41) respectively](Table 2). Compared with participants with normal LVEF, low LVEF was associated with increased risk of all-cause death in the unadjusted model, after adjusting for age, sex and race/ethnicity and also in our full multivariable model[HR(95%CI): 3.32(2.16 – 5.10), 2.69(1.75-4.14) and 3.03(1.94-4.73) respectively](Table 2).The adjusted cubic spline analysis with the LVEF 55% as the reference showed a non-linear relationship between increasing LVEF and hazard ratios. The LVEF least associated with future CHF was approximately 62.5%. LVEF of 55% and approximately 70% have similar risk for future CHF. Individuals with LVEF less than 55% and those with LVEF greater than approximately 70% have an increased risk for CHF compared with those with LVEF =55%.
Time to incident heart failure for low, low normal, and normal ejection fraction (log rank p – value <0.001)
Table 2
Association of low and low normal ejection fraction with incident congestive heart failure and mortality.
| Hazard Ratio (95% CI)
|
||||
|---|---|---|---|---|
| Unadjusted | Model1‡ | Model 2δ | ||
|
|
||||
| Events/Total at Risk | Risk of Incident Heart Failure | |||
|
|
||||
| Normal EF | 109/4742 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Low normal EF | 8/101 | 3.58 (1.74, 7.33) | 3.57 (1.74, 7.34) | 3.64 (1.76, 7.52) |
|
|
||||
| Low EF | 19/83 | 13.31 (8.17,17.33) | 11.34 (6.87, 18.71) | 9.52 (5.63, 17.52) |
|
|
||||
| Risk of All-cause Death | ||||
|
|
||||
| Normal EF | 427/4742 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Low normal EF | 11/101 | 1.21 (0.66, 2.19) | 1.23 (0.68, 2.24) | 1.32 (0.72, 2.41) |
|
|
|
|||
| Low EF | 22/83 | 3.32 (2.16, 5.10) | 2.69 (1.75, 4.14) | 3.03 (1.94, 4.73) |
|
|
|
|||
*Model 1: Adjusted for age, sex, race
Discussion
The goal of this study was to assess the prevalence and prognosis of asymptomatic population based adults with low normal LVEF; a subgroup who will not be considered as having stage B heart failure by the AHA HF classification unless they have associated structural heart disease such as LVH, significant valvular heart disease or MI. Our study show that low normal LVEF is as prevalent as low LVEF in asymptomatic population based adults and is an independent predictor for future CHF even after adjusting for LV mass. The risk of low normal LVEF for incident CHF compared with normal LVEF was significant enough (> 3 times) to support future preventive therapy considerations.
The AHA heart failure classification was developed to include early stages (A&B) of heart failure for preventive therapy1. Stage B HF includes individuals with structural heart disease but without signs and symptoms of heart failure1,2,8. This includes patients with prior myocardial infarction, left ventricular remodeling including left ventricular hypertrophy and low left ventricular ejection fraction and significant valvular heart disease. For such individuals the AHA recommends drugs such as ACE-inhibitors, angiotensin receptor blockers and beta-blocker therapies if appropriate to reduce the progression to clinical heart failure (stage C&D)12–14. Even though the AHA heart failure classification document does not explicitly provide a cut off for low left ventricular ejection fraction, in clinical practice low normal LVEF (50-55%) is not included in this classification. Our prior study15 showed that individuals with asymptomatic left ventricular systolic dysfunction (ASLVD) defined as LVEF <50% had ~9 times the risk for future CHF compared with those with LVEF≥ 50%, and hence supported the therapeutic recommendations enumerated by the AHA HF guidelines to reduce this risk. The present study suggests that within those with LVEF ≥ 50%, those with low normal LVEF have > 3 times the risk of being diagnosed with CHF compared with those with LVEF >55% over a 10 year period and may warrant inclusion in future preventive HF clinical trials.
There are several mechanisms by which the LV myocardium can be damage leading to heart failure16. These mechanisms can be broadly divided into ischemic, due to coronary artery disease/ myocardial infarction and non-ischemic, which includes a multitude of courses16. Our study excluded participants who had adjudicated myocardial infarction during the follow up period and hence any myocardial damage that precipitated CHF in our study may be considered as non-ischemic in origin. Regardless of the mechanism of myocardial damage, if the pathological outcome is a reduction in LVEF, current data suggests that chronic therapy with ACE inhibitors and beta blockers reduces the progression to clinic heart failure 12–14. The conundrum is the definition of reduced LVEF. Our study did not assess left ventricular diastology and fibrosis17,18 but suggests that individuals with LVEF 50-55% may have already had myocardial damage and are on their way to clinical heart failure. Initiating preventive therapy in appropriate asymptomatic individuals with low normal LVEF may amount to early interruption of the pathological process and may translate into significant reduction in clinical heart failure in our communities.
Our study suggests a U shape association between the risk of future CHF and LVEF in asymptomatic individuals with LVEF≥50%, with the least future CHF risk corresponding to LVEF of approx. 62.5%. The two arms of the U shape may represent the risk of HFref and HFpef. Thus our effort of preventing future CHF in asymptomatic individuals should target 2 distinct types of risk (risk for HFref and HFpef). Presently there are no known preventive strategies for HFpef. Validation of this finding in other cohorts is needed.
The present study has several limitations. First MESA exam 1 cardiac MRI did not assess diastology and myocardial fibrosis. Diastology and the degree of myocardial fibrosis in this cohort are therefore unknown. It is therefore possible that low normal LVEF may qualify as stage B (having structural heart disease due to abnormal diastology or significant fibrosis). The current analysis also did not account for change in medications use, especially ACE-I, ARBs and beta blockers, during the follow up period. This may have affected our clinical CHF event rate. MESA did not use echocardiography, the predominant modality used in clinical practice, to assess left ventricular ejection fraction during the baseline exam. Unlike cardiac MRI, echocardiography has been shown to have higher inter- and intra-observer variability. It is unclear how using echocardiography instead of cardiac MRI would affect our results and conclusions. Replication of our findings in asymptomatic population based adults using echocardiography as the imaging modality for assessing left ventricular ejection fraction is needed. We did not stratify our analysis by type of CHF (HFref and HFpef) due to the relatively small number of MESA participants with available data on LVEF at CHF diagnosis. Finally even though we adjusted for several relevant factors, our results may still be due to residual confounding.
Conclusion
Low normal LVEF is as prevalent as low LVEF in asymptomatic community dwelling adults. We observed a gradient-response association between LVEF categories (Low, low normal and normal) and incident CHF; individuals with low normal LVEF having >3 times risk compared with normal LVEF. Current CHF preventative efforts should also target individuals with low normal LVEF. Validation of our findings is needed.
Non-Linear Association of ≥50% left ventricular ejection fraction with Incident Congestive Heart Failure (Hazard Ratio is in reference to left ventricular ejection fraction of 55%)
Highlights
-
Low normal LVEF is as prevalent as low LVEF in asymptomatic community dwelling adults
-
Low normal LVEF has >3 times increased risk for future CHF compared with normal LVEF
-
There is a gradient-response association between low, low normal, normal LVEF and risk of future CHF in asymptomatic adults
-
Future CHF preventive strategies should also target individuals with low normal LVEF to assess if this risk could be reduced.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR
Footnotes
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None